Advanced
Meetings & conferences
HealthIn vitroAdvanced
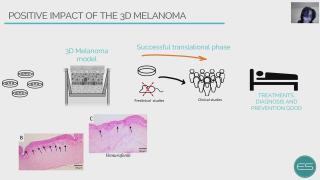
A 3D melanoma for the development of skin cancer treatment
The developing process of a new drug, from first testing to regulatory approval and ultimately to market is a long, costly, and risky path. Noteworthy is the fact that almost 95% of the drugs that go into human trials fail. According to the National Institutes of Health (NIH), 80 to 90% of drug research projects fail before they ever get tested in humans. The value of preclinical research, mainly conducted in animal model experiments for predicting the effectiveness of therapies and treatment strategies in human trials, has remained controversial. Only 6% of the animal studies are successfully translated into the human response. Breaking down failure rates by therapeutic area, oncology disorders account for 30% of all failures. The absence of human-relevant models with receptors, proteins, and drug interactions in the in situ microenvironment leaves a gap in the scientific discovery process of new therapies. In this context, the present work presents the development of a sophisticated in vitro skin model platform focus on boosting melanoma treatment. The results showed a physiological microenvironment of human skin with epidermal differentiation and development of stratified layers (basement membrane, stratum spinosum, stratum granulosum, and stratum corneum). Furthermore, it was observed the pathophysiological microenvironment of the melanoma with invasion or migration through the basement membrane into the dermis and no epidermal differentiation. Vemurafenib treatment, the gold standard which targets BRAF mutations, showed a decrease in proliferation and invasion of melanoma tumors, with an increase in epidermis keratinization. Melanoma incidence continues to increase year-on-year and is currently responsible for >80% of skin cancer deaths. It is the most common cutaneous form and is known to have the highest mutational load of all cancers. Nowadays, patients with advanced melanoma BRAFV600E mutation can benefit from monotherapies or targeted therapies. Although the initial response rate is effective, disease progression and tumor chemoresistance rapidly occur in the majority of patients. Therefore, the treatment of melanoma remains a challenge, and despite the advances, there is still an urgent need to identify new therapeutic strategies. 3D Model Melanoma is considered one important tool for studying the evolution of the pathology, as well as evaluating the effectiveness of new therapeutic approaches.
Meetings & conferences
HealthIn vitroAdvanced
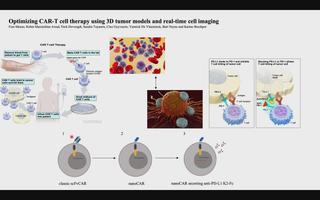
3D tumor models for CAR-T-cell therapy optimization
Chimeric antigen receptor (CAR) T-cell therapy accounts for one of the most promising therapeutic advances in cancer immunotherapy. In this form of adoptive cell transfer, T-cells of a patient are engineered to express so-called ‘CARs’, in which the antigen-recognition capacity of antibodies is combined with T-cell activating domains. So far, CAR-T-cell therapy obtained its most impressive results in hematological malignancies resulting in the approval of five CAR-T cell products by the FDA for hematologic indications. However, CAR-T-cell therapy has not mirrored its success in solid tumors. The poor efficacy of CAR-T-cell therapy in solid tumors has, in part, been attributed to the lack of understanding in how CAR-T-cells function in a solid tumor microenvironment. Classical validation methods rely on the use of specificity and functionality assays in 2D models against adherent target cells or target cells in suspension. Yet, by using these models, observations made in vitro may differ greatly to an in vivo situation where tumors are engrafted in 3D structures. We developed a more relevant and translational 3D tumor model using eGFP+ target cells. This allows us to couple 3D tumor cell killing by CAR-T-cells to live-cell imaging, providing an efficient quantification of target cell death. As proof- of-concept, we used a 3D model of eGFP+ glioblastoma cells and CAR-T-cells targeting a pan-cancer antigen. This 3D glioblastoma model allowed us to show that classical scFv-based CAR-T-cell therapy of glioblastoma cells can be improved by nanoCAR-T-cells. Furthermore, combining nanoCAR-T-cell therapy with a genetic approach of nanobody-based anti-PD-L1 immune checkpoint blockade further increased the cytotoxicity of the nanoCAR-T-cell therapy.
Meetings & conferences
ToxicologyIn vitroAdvanced
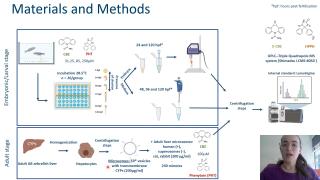
Biotransformation of proteratogenic anti-epileptics in the zebrafish embryo
The zebrafish (Danio rerio) embryo has gained interest as an alternative model for developmental toxicity testing, which still mainly relies on in vivo mammalian models (e.g., rat, rabbit). However, cytochrome P450 (CYP)-mediated drug metabolism, which is critical for the bioactivation of several proteratogens, is still under debate for this model. Therefore, we investigated the potential capacity of zebrafish embryos/larvae to bioactivate two known mammalian proteratogens, carbamazepine (CBZ) and phenytoin (PHE) into their mammalian active metabolites, carbamazepine-10,11-epoxide (E-CBZ) and 5-(4-hydroxyphenyl)-5-phenylhydantoin (HPPH), respectively. Zebrafish embryos were exposed to three concentrations (31.25, 85, and 250 μM) of CBZ and PHE from 51⁄4 to 120 hours post fertilization (hpf) at 28.5°C under a 14/10 hour light/dark cycle. For species comparison, also adult zebrafish, rat, rabbit and human liver microsomes (200 μg/ml) were exposed to 100 μM of CBZ or PHE for 240 minutes at 28.5°C. Potential formation of the mammalian metabolites was assessed in the embryo medium (48, 96, and 120 hpf); pooled (n=20) whole embryos/larvae extracts (24 and 120 hpf); and in the microsomal reaction mixtures (at 5 and 240 minutes) by targeted investigation using a UPLC–Triple Quadrupole MS system with lamotrigine (0.39 μM) as internal standard. Our study showed that zebrafish embryos metabolize CBZ to E-CBZ, but only at the end of organogenesis (from 96 hpf onwards), and no biotransformation of PHE to HPPH occurred. In contrast, our in vitro drug metabolism assay showed that adult zebrafish metabolize both compounds into their active mammalian metabolites. However, significant differences in metabolic rate were observed among the investigated species. These results highlight the importance of including the zebrafish in the in vitro drug metabolism testing battery
for accurate species selection in toxicity studies.
Meetings & conferences
HealthIn vitroAdvanced
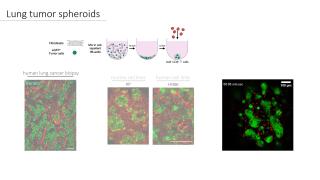
Lung tumor spheroids for onco-immunological research
Lung cancer thrives in a complex multicellular tumor microenvironment that impacts tumor growth, metastasis, response, and resistance to therapy. While orthotopic murine lung cancer models can partly recapitulate this complexity, they do not resonate with high-throughput immunotherapeutic drug screening assays. To address the current need for relevant and easy-to-use lung tumor models, we established a protocol for fully histo-compatible murine and human lung tumor spheroids, generated by co-culturing lung fibroblasts with tumor cells in ultra-low adherence 96-well plates. Moreover, we describe their application potential to study tumor-stroma organization, T-cell motility, and infiltration as well as distinct macrophage subsets’ behavior using confocal microscopy. Finally, we report on a 3D target specific T-cell killing assay that allows spatio-temporal assessment using live cell imaging and flow cytometry. This lung tumor spheroid platform can serve as a blueprint for other solid cancer types to comply with the need for straightforward onco-immunology assays.
Meetings & conferences
HealthIn vitroAdvanced
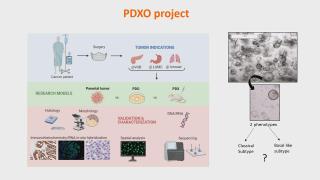
Characterizing pancreatic cancer with omics
Pancreatic ductal adenocarcinoma (PDAC) is known for its aggressive biology and lethality. Due to a low success rate of current diagnostic and therapeutic approaches in clinic, there is an urgent need for preclinical research studies to investigate the underlying biology of this malignancy. This knowledge is indispensable to facilitate the development and validation of potential new therapeutic compounds. Superior to conventional biomedical research models, the focus of this study is on the development and use of a well-established patient-derived 3D in vivo model, mimicking the tumor as it is present in a human body. The development and characterization of pancreatic cancer derived organoids. This model is extensively analysed using advanced histological methods omics technology to perform tumor subtyping. 15 established PDAC organoid lines and their corresponding parental tumors are validated using immunostainings and DNA hotspot sequencing. This study is the first to show in situ detection of important driver mutations of pancreatic cancer, like KrasG12D, both in parental tumor and organoids. Additionally, specific culture conditions are defined to develop subtype-specific organoids which are validated using multiplex RNA in situ hybridization and transcriptomics. We are proud to collaborate in a fruitful international project, aiming to set-up a pre-clinical screening platform for pancreatic cancer based on patient-derived organoids -and xenografts. Altogether, spatial-omics in depth analysis of both models will demonstrate (1) high resemblance to parental tissue and (2) subtype-specific signatures associated with type of model. Ultimately, the screening platform can be used by pharmaceutical companies to facilitate oncological drug testing in a subtype specific way.
Publications Ilse Rooman's lab:
https://pubmed.ncbi.nlm.nih.gov/34330784/
https://pubmed.ncbi.nlm.nih.gov/31161208/
Meetings & conferences
HealthAdvanced
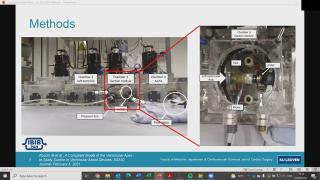
An in vitro simulator for ventricular assist devices
Despite the positive outcomes of ventricular assist device (VAD) therapy, there are still some adverse events, such as suction, caused by the mismatch of the preload of the left ventricle and the flow of the pump. Ventricular suction can be due to multiple underlying pathophysiological conditions. However, the medical management of such patients is still not well defined. In order to analyse the potential of different therapeutic interventions to mitigate suction in different pathophysiological conditions, a suction module is embedded in a cardiovascular hybrid (hydraulic-computational) simulator. The suction module mimics the ventricular apex and its connection with a HeartWare HVAD system (Medtronic). With such a set-up, the medical management of different pathophysiological conditions can be evaluated, without the use of animals.
Also watch the video on the cardiovascular simulator used for this research: https://tpi.tv/watch/76
Contact: https://www.linkedin.com/in/maria-rocchi-9aa7b4162/
Meetings & conferences
HealthIn vitroAdvanced
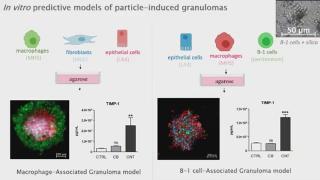
In vitro predictive models of particle-induced granulomas
Léa Hiéronimus is a PhD student at the Louvain centre for Toxicology and Applied Pharmacology (LTAP, UCLouvain, Belgium). Léa is working in François Huaux's team, where we are trying to better understand how certain inhaled particles exert their toxicity. The goal is to better diagnose and treat individuals exposed to particles, but also to identify the particle characteristics which induce, or do not induce, toxic effects. For this, Léa studies a very particular cell type which seems to be involved in particle responses. Indeed, we have found the specific accumulation of the innate subset of B-lymphocytes called “B-1 lymphocytes”, which occurred during granuloma formation/maturation induced by inhaled particles in mice. According to the literature, this accumulation can be attributed to their migration from mesothelial cavities such as the peritoneum, acting as a reservoir.
In addition to conventional particles-induced granulomas, which formation rely on macrophages responses, we developed new models relying on B-1 lymphocytes. Indeed, B-1 lymphocytes show a unique clustering property, that is not observed using macrophages or other subsets of B-lymphocytes (conventional B-2 lymphocytes) as purified B-1 lymphocytes regroup granuloma-inducing particles (carbon nanotubes CNT7, crocidolite asbestos, micrometric silica MinUSil and MSS, cobalt oxide,…) but not carbon black, a particle not-inducing granuloma in vivo. Additionally, we developed a model aiming to recapitulate the lung after B-1 lymphocytes migration and found that macrophages and epithelial cells (MHS and LA4 cell lines) where grouped to form spheroids when in coculture with B-1 and not B-2 lymphocytes.
These models will serve as tools to identify new mediators of granuloma formation, which could serve as biomarkers and/or therapeutic targets for exposed individuals. On the other hand, we aim to propose new bioassays for the prediction of granuloma-inducing materials using alternative models.
Lab website: https://uclouvain.be/en/research-institutes/irec/ltap
Contact: lea.hieronimus@uclouvain.be
Meetings & conferences
HealthToxicologyIn vitroAdvanced
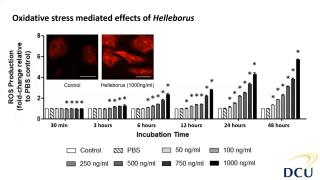
Characterisation and cytotoxicity assessment of Helleborus with NAMs
Helleborus sp. is a member of the Ranunculaceae family, and are small, perennial herbs common in Central and Southern Europe and Asia. Their distribution in Europe elevated their position in therapeutic remedies since the ancient time and mythology. Due to their potent and rich extracts from their roots, hellebores have been used in traditional and folklore remedies as they present rich sources in glycosides. Mainly, these plants have exhibited cathartic, anthelmintic and other beneficial aspects to treat diseases, however, hellebores have also been known for their adverse and poisonous aspects. It is also because of their cytotoxic aspect that these species have also been explored as alternative approaches to cancer treatment and are mainly reported as sporadic patient cases in literature. In this study, we first focused on the phytochemical characterisation of Helleborus odorus subsp. cyclophyllus combining biochemical assays and a detailed characterisation of its antioxidant and antibacterial properties. Furthermore, regarding its toxic potential, we explored the cytotoxic toxic properties and the mechanisms of toxicity mediated effects using in vitro cell systems primary human aortic endothelial cells (HAECs). HAECs are useful for studying vascular diseases such as thrombosis, atherosclerosis, and hypertension as well as for stent-graft compatibility testing and within the 3Rs principles, avoiding animals in these studies. Results showed the cytotoxic and reactive oxygen species potential of Helleborus extract in dose and time dependent manner. Further investigation (not shown here) revealed more mechanistic effects relevant to inhibition of proliferation.
Contact: https://www.researchgate.net/profile/Anna-Michalaki
Meetings & conferences
HealthIn vitroAdvanced
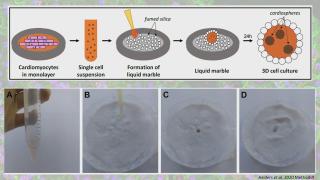
Liquid marbles for cardiac organoids development
Advances in three-dimensional (3D) culture techniques have shown several advantages over 2D cultures, especially by more accurately mimicking the in vivo environment. This has led to improved reproducibility and reliability of experimental results, which are important criteria in disease modelling and toxicity testing. Induced pluripotent stem cells (iPSC) provide an unlimited source for the derivation of all cell types of the adult body, including cardiomyocytes. To improve the current culture methods for multicellular cardiac spheroids, such as the hanging drop method, we explored the use of hydrophobic powders. Fumed silica nanoparticles can be used to encapsulate liquid drops, which could serve as a microenvironment for cell cultures. This microbioreactor stimulates cell coalescence and 3D aggregation while providing optimal gas exchange between the interior and the surrounding environment. Moreover, the properties of liquid marble microbioreactors render them ideal for co-culture experiments. This liquid marble technique has been previously explored and optimized for other cell types. Here we describe a protocol that allows for the derivation of functional cardiac mini organoids, consisting of co-cultured cardiomyocytes and cardiac fibroblasts. These cardiospheres can be valuable for modelling cardiac diseases in vitro and assessing cell interactions to decipher disease mechanisms.
Lab website: https://www.medicalcellbiologylab.com/
Contact: https://www.researchgate.net/profile/Jeffrey-Aalders
RE-place database: https://www.re-place.be/method/liquid-marbles-cost-effective-platform-generate-cardiospheres-co-cultured-cardiomyocytes-and
Meetings & conferences
HealthIn vitroIn silicoAdvanced
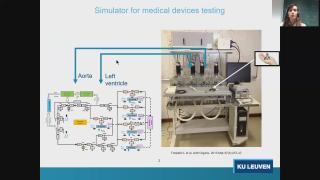
A hybrid in silico-in vitro cardiorespiratory simulator for medical device testing
Cardiovascular medical devices (CMDs) (e.g. artificial hearts, ventricular assist devices, ECMO, heart valves) support the cardiac and/or the respiratory function of patients. Large challenges are encountered when assessing CMDs interaction with the human body and the effects on the heart and vessels. Especially CMDs with new designs require an extensive evaluation concerning their effectiveness and safety under different pathophysiological conditions. We propose a high fidelity cardiorespiratory simulator for the testing of the hemodynamic performance of CMDs. The proposed simulator merges the flexibility of the in silico system with a hydraulic interface to test CMDs. As such, the simulator embeds a high fidelity cardiorespiratory model, allowing the reproduction of pathologies at both cardiac and respiratory level. The simulator works as a test bench for the assessment of CMDs, from prototype stage to pre-clinical stage. Thanks to its flexibility and high-fidelity, the simulator helps reducing animal testing and provides insights on how to improve CMD design to better suit different patient’s needs.
Contact: https://www.kuleuven.be/wieiswie/en/person/00098489
RE-place database: https://www.re-place.be/method/cardiovascular-modelling-medical-device-testing
Meetings & conferences
ToxicologyIn vitroAdvanced
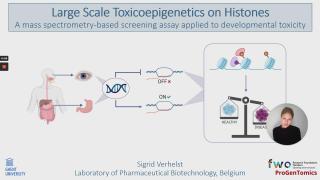
Toxicoepigenetics on histones using mass spectrometry
Toxicoepigenetics is an emerging field that studies the toxicological impact of compounds on protein expression through heritable, non-genetic mechanisms, such as histone post-translational modifications (hPTMs). Due to substantial progress in the large-scale study of hPTMs, integration into the field of toxicology is promising and offers the opportunity to gain novel insights into toxicological phenomena. Moreover, there is a growing demand for high-throughput human-based in vitro assays for toxicity testing, especially for developmental toxicity. Consequently, we developed a mass spectrometry-based proof-of-concept to assess a histone code screening assay capable of simultaneously detecting multiple hPTM-changes in human embryonic stem cells. To prove the applicability and performance, we first validated the untargeted workflow with valproic acid (VPA), a histone deacetylase inhibitor. These results demonstrate that our workflow is capable of mapping the hPTM-dynamics, with a general increase in acetylations as an internal control. To illustrate the scalability, a dose-response study was performed on a proof-of-concept library of ten compounds i) with a known effect on the hPTMs (BIX-01294, 3-Deazaneplanocin A, Trichostatin A, and VPA), ii) classified as highly embryotoxic by the European Centre for the Validation of Alternative Methods (ECVAM) (Methotrexate, and All-trans retinoic acid), iii) classified as non-embryotoxic by ECVAM (Penicillin G), and iv) compounds of abuse with presumed developmental toxicity (ethanol, caffeine, and nicotine). In conclusion, we show that toxicoepigenetic screening on histones is feasible and yields very rich data that can contribute to the (safe) development of drugs and holds potential, not only for applications in the pharmaceutical industry, but also for environmental toxicity and food safety.
Lab website: https://www.progentomics.ugent.be/
Linkedin: https://www.linkedin.com/in/sigrid-verhelst-a54798172/
ResearchGate: https://www.researchgate.net/profile/Sigrid-Verhelst